The First Light
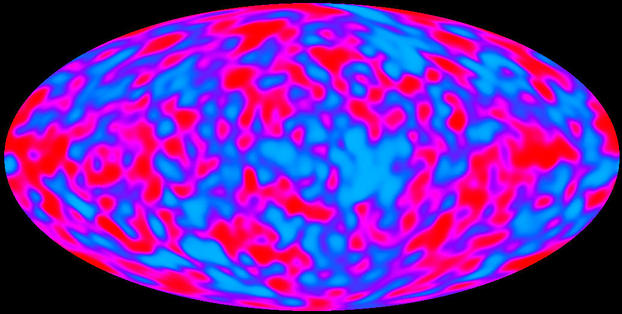 A flattened globe represents the inside view of the skies over-head. No matter what direction we look, the temperature of the first light is almost the same. False color represents the temperature fluctuations. The red regions are about 40 microkelvins warmer than the blue regions.
A flattened globe represents the inside view of the skies over-head. No matter what direction we look, the temperature of the first light is almost the same. False color represents the temperature fluctuations. The red regions are about 40 microkelvins warmer than the blue regions.
Researchers have by now studied the first light with antennas even larger than the one Penzias and Wilson used. The Earth’s atmosphere absorbs a large part of the signal. To see better, experimenters worked with mountain-top and balloon-borne antennas. In 1989 NASA launched the Cosmic Background Explorer (COBE). In outer space this satellite was far from human-made interference on Earth. It was also outside the atmosphere. From there the instruments studied the electromagnetic waves that Penzias and Wilson called the “background.” When the waves started out, they were ordinary light, perhaps a little more reddish than sunlight. For this reason, the experimenters may present their data as a “photograph.”
More recently WMAP has “photographed” the first light with higher resolution and refined the COBE satellite picture.
Heat and light produced the pressure that drove the expansion. Like the original gamma rays, the newly materialized particles also moved in all directions at various speeds. This kind of movement, entirely due to heat and completely random, is what we call thermal agitation. Its pressure depends only on the temperature.
People are familiar with relative temperature, measured above or below the freezing point of water on the centigrade or Celsius scale, or above or below the temperature of a well-drained salt-and-ice mixture on the Fahrenheit scale. Scientists measure absolute temperature from the lowest possible temperature, a temperature so low that thermal agitation ceases and particles are frozen together. The lowest possible temperature is called “absolute zero.” On the more familiar scales it is ‑273.15º C or ‑459.67º F.
Scientists no longer use the word “degrees” when quoting an absolute temperature. The unit of absolute temperature is the kelvin, after William Thomson Kelvin (British mathematician and physicist, 1824–1907). The kelvin is a unit of temperature just as the meter is a unit of distance and the second is a unit of time. We do not say “degrees meter” or “degrees second.” On the absolute temperature scale water freezes at +273.15 kelvins (0º C, 32º F) and boils at +373.15 kelvins (100º C, 212º F). Kelvins and Centigrade degrees represent the same increment of temperature, but a Fahrenheit degree is 5/9ths of that increment.
Since the mixture was hot and thermal, physicists can apply to it everything they know from studying similar mixtures in the laboratory. This warrants their theoretical analyses, and builds confidence that we really understand what we are seeing.
The characteristics of thermal agitation are well known from theoretical analysis and experimental confirmation. Temperature determines most of its characteristics.
Robert Brown (British botanist, 1773–1858) used a microscope to observe small particles suspended in a liquid. He discovered that they move erratically. In 1905 Einstein explained that the erratic motion arises because random thermal agitation of the molecules of the liquid makes pressure fluctuations. The net pressure is slightly higher first on one side of a small particle, then on another. Experimentalists subsequently confirmed Einstein’s explanation.
Thermal agitation pressure is not entirely steady. It comes from a random bombardment of particles. Since the bombardment is random, the pressure has small fluctuations. Fluctuations are the visible result of thermal agitation. The light from the early universe is the most perfectly thermal light physicists have ever analysed. The fluctuations are the evidence that the background Penzias and Wilson detected really is the first light.
The universe as it is now could not have formed from a perfectly uniform initial state. There can be no net gravitational attraction if all the material and energy is evenly distributed over all points of space. Gravitational force is directly proportional to the mass of material and the equivalent mass of the energy at a given point. If the total mass of material and energy is the same at every point, then the attraction from any point exactly counterbalances the attraction from all other points.
If two teams playing a tug-of-war are equally strong there can be no movement provided the rope is stronger than the teams.
Fluctuations, however, let some regions pull harder than others. The regions that pulled hardest were the densest regions, those with the most material and energy packed into them. They attracted material and energy out of the more rarefied regions. The movement of attracted material and energy left the dense regions denser and the rarefied regions more rarefied. This made the fluctuations more pronounced. It also made the dense regions more strongly attracting.
The original dense fluctuations served as seeds of condensation or centers of attraction. The gravity of the dense regions compressed them and heated them up again. It also swept space bare and made the rarefied regions more rarefied. Eventually the rarefied regions joined together, forming a dark, cold void punctuated with dense clouds of bright, hot gas. The dense clouds became galaxies containing stars and planets. The universe took on the aspect we see now in the night sky: isolated points of light and heat in a dark void.
More recently WMAP has “photographed” the first light with higher resolution and refined the COBE satellite picture.
Heat and light produced the pressure that drove the expansion. Like the original gamma rays, the newly materialized particles also moved in all directions at various speeds. This kind of movement, entirely due to heat and completely random, is what we call thermal agitation. Its pressure depends only on the temperature.
People are familiar with relative temperature, measured above or below the freezing point of water on the centigrade or Celsius scale, or above or below the temperature of a well-drained salt-and-ice mixture on the Fahrenheit scale. Scientists measure absolute temperature from the lowest possible temperature, a temperature so low that thermal agitation ceases and particles are frozen together. The lowest possible temperature is called “absolute zero.” On the more familiar scales it is ‑273.15º C or ‑459.67º F.
Scientists no longer use the word “degrees” when quoting an absolute temperature. The unit of absolute temperature is the kelvin, after William Thomson Kelvin (British mathematician and physicist, 1824–1907). The kelvin is a unit of temperature just as the meter is a unit of distance and the second is a unit of time. We do not say “degrees meter” or “degrees second.” On the absolute temperature scale water freezes at +273.15 kelvins (0º C, 32º F) and boils at +373.15 kelvins (100º C, 212º F). Kelvins and Centigrade degrees represent the same increment of temperature, but a Fahrenheit degree is 5/9ths of that increment.
Since the mixture was hot and thermal, physicists can apply to it everything they know from studying similar mixtures in the laboratory. This warrants their theoretical analyses, and builds confidence that we really understand what we are seeing.
The characteristics of thermal agitation are well known from theoretical analysis and experimental confirmation. Temperature determines most of its characteristics.
Robert Brown (British botanist, 1773–1858) used a microscope to observe small particles suspended in a liquid. He discovered that they move erratically. In 1905 Einstein explained that the erratic motion arises because random thermal agitation of the molecules of the liquid makes pressure fluctuations. The net pressure is slightly higher first on one side of a small particle, then on another. Experimentalists subsequently confirmed Einstein’s explanation.
Thermal agitation pressure is not entirely steady. It comes from a random bombardment of particles. Since the bombardment is random, the pressure has small fluctuations. Fluctuations are the visible result of thermal agitation. The light from the early universe is the most perfectly thermal light physicists have ever analysed. The fluctuations are the evidence that the background Penzias and Wilson detected really is the first light.
The universe as it is now could not have formed from a perfectly uniform initial state. There can be no net gravitational attraction if all the material and energy is evenly distributed over all points of space. Gravitational force is directly proportional to the mass of material and the equivalent mass of the energy at a given point. If the total mass of material and energy is the same at every point, then the attraction from any point exactly counterbalances the attraction from all other points.
If two teams playing a tug-of-war are equally strong there can be no movement provided the rope is stronger than the teams.
Fluctuations, however, let some regions pull harder than others. The regions that pulled hardest were the densest regions, those with the most material and energy packed into them. They attracted material and energy out of the more rarefied regions. The movement of attracted material and energy left the dense regions denser and the rarefied regions more rarefied. This made the fluctuations more pronounced. It also made the dense regions more strongly attracting.
The original dense fluctuations served as seeds of condensation or centers of attraction. The gravity of the dense regions compressed them and heated them up again. It also swept space bare and made the rarefied regions more rarefied. Eventually the rarefied regions joined together, forming a dark, cold void punctuated with dense clouds of bright, hot gas. The dense clouds became galaxies containing stars and planets. The universe took on the aspect we see now in the night sky: isolated points of light and heat in a dark void.
Separating Light from Darkness
Our kind of life depends on complex arrangements of atoms. Atoms cannot stick together at temperatures of millions, or even thousands, of degrees. But when the first atoms formed, they were still hot, and they spread nearly uniformly throughout the universe. If the temperature, pressure, and density were always perfectly uniform, there would never have been a habitable place in the universe. Life’s habitat must have moderate temperatures, those that make water liquid, neither frozen as ice nor boiled into steam. But a much hotter source must supply life with light as well as heat. A perfectly uniform universe cannot have both a star like the Sun and a planet with moderate temperatures like the Earth.
Happily for us, the universe was not perfectly uniform. There were fluctuations of temperature, pressure, and density. These fluctuations had to exist if the fiery mixture was to separate into concentrated regions, some hot enough to supply light and others at moderate temperatures where atoms could form the complex arrangements that life requires. The light had to separate from the darkness.
Our kind of life depends on complex arrangements of atoms. Atoms cannot stick together at temperatures of millions, or even thousands, of degrees. But when the first atoms formed, they were still hot, and they spread nearly uniformly throughout the universe. If the temperature, pressure, and density were always perfectly uniform, there would never have been a habitable place in the universe. Life’s habitat must have moderate temperatures, those that make water liquid, neither frozen as ice nor boiled into steam. But a much hotter source must supply life with light as well as heat. A perfectly uniform universe cannot have both a star like the Sun and a planet with moderate temperatures like the Earth.
Happily for us, the universe was not perfectly uniform. There were fluctuations of temperature, pressure, and density. These fluctuations had to exist if the fiery mixture was to separate into concentrated regions, some hot enough to supply light and others at moderate temperatures where atoms could form the complex arrangements that life requires. The light had to separate from the darkness.